Jelly rolls and coking drums: a bottom-up look at batteries 🔋

I’ve spent a lot of the past few months thinking about batteries, which turn out to be one of the most fascinating topics in modern economics. Here’s a film I’ve made for Sky News about it. Please do watch it!
The main problem I had while researching and reporting on this topic was the best kind of problem: there is so much fascinating content it’s hard to include everything, either in the film or indeed the very long long read I wrote for the Sky website.
There’s the 30,000ft questions about their part in the energy transition. There’s the questions about where we’ll get all the raw materials we need for these batteries. The scale of this is somewhat astounding, as you’ll see from these data I recently reported about for Sky News, privately submitted to an internal UK government committee.

The blue bars (if you can make them out) show the amount of these substances which the UK currently needs for its battery production. The red bars show you how much we’ll need by 2030 if we’re going to have a gigafactory business capable of supporting the UK car industry as it currently exists. We are talking about staggering increases - 9,000% or more. (Interestingly, the projected increase in cobalt is less than for the other materials, because battery makers are getting better and better at whittling down the cobalt content of their battery chemistries).
The figures above are predicated on another chart, this one showing the amount of battery production the UK might plausibly be heading for by 2030, the year it bans the sale of new internal combustion engine cars.
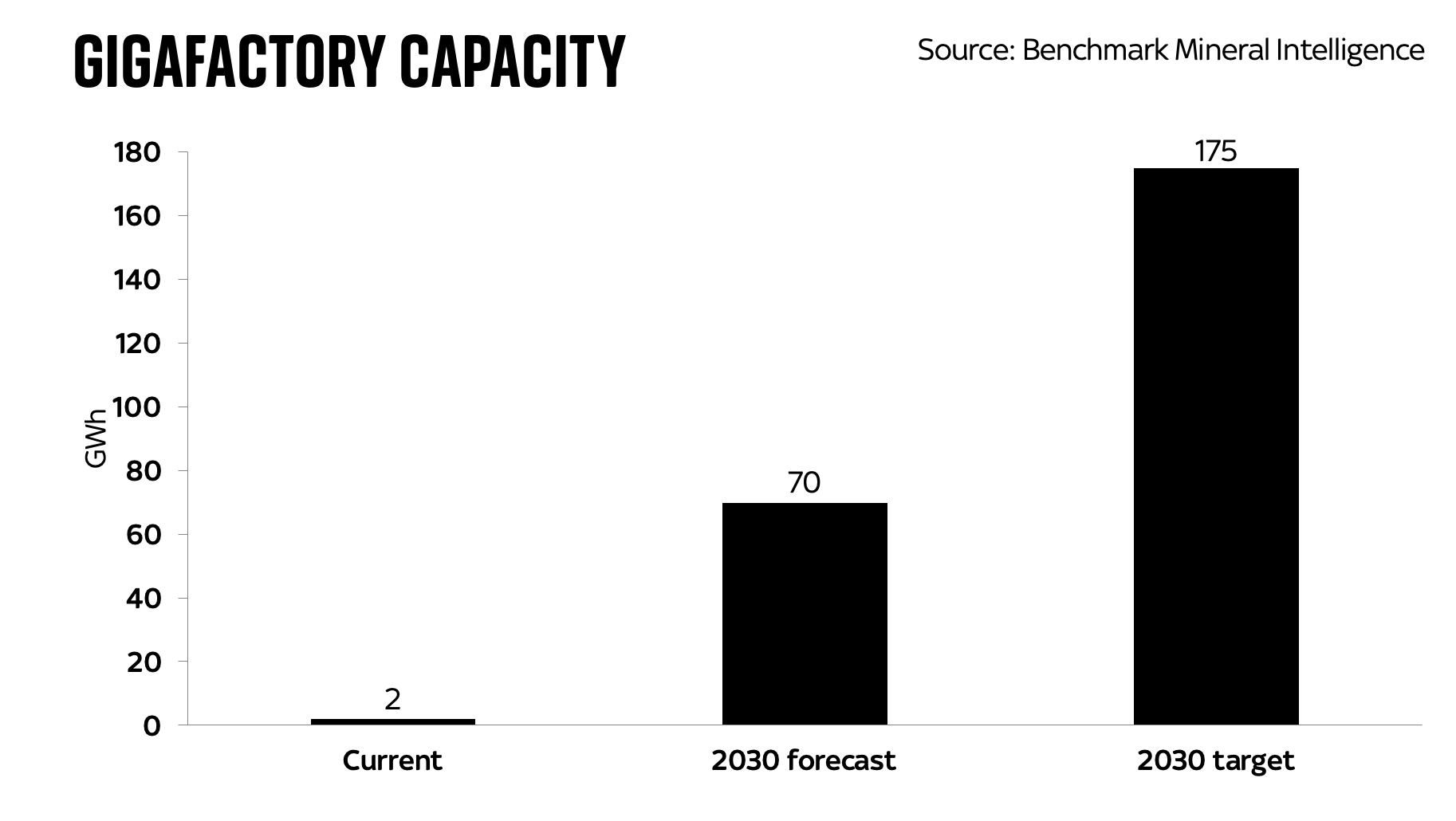
The right hand bar is what we produce now, just under 2 gigawatt-hours at the Nissan/Envision plant. The middle bar is what happens if the government’s current plans work out (though it would actually like to get that bar up a bit further, perhaps to 90GWh). But the real thing to take note of is the bar on the right: where the UK needs to be in terms of battery production by 2030 if it’s planning to replace the roughly 2-3m unit production we have today. And note that in one sense this is an underestimate, given the above is purely for the most common lithium ion batteries (NMC). The analysts behind this data think we need a further 100GWh of LFP battery production (LFP being a slightly different chemistry, one which is slightly safer but has slightly shorter range).
Anyway I’m getting sidetracked. There’s the scale of materials, the scale of industrial capacity needed. There’s the question of where those battery materials come from. And while countless column inches have been written about lithium and cobalt and (to a lesser extent) nickel - for good reason, one thing that struck me throughout my research is how often the other materials in a battery are neglected - especially the graphite that forms the anode.

That brings us to this place: the Humber Refinery on the coast of the North Sea in England. It looks and smells like a ghost of fossil fuel history. It's where they turn crude oil into petrol, jet fuel and many other petrochemical products. So not the obvious place to visit if you’re interested in batteries.
Before we head in, it’s worth noting the basic chemistry of a battery, which isn't immediately obvious from the outside. Take a typical cylindrical lithium-ion battery. A Tesla is a big slab of thousands of these batteries with a car on top. There are other types of batteries; prismatic and pouch for instance and one could go down a fascinating rabbit hole on that topic. But for the time being let’s look inside the battery.

Now the shape of these batteries is actually a bit misleading. They're not, as you might have thought, canisters of liquid with positive electrode at top and negative at bottom. There is some liquid electrolyte in there providing a kind of electrochemical lubrication, but the real action, the most important element of the battery are thin films of metal, covered in special chemicals and rolled up into a spiral. They call them jelly rolls (in the UK we’d say “sponge rolls”). The term isn’t a nickname; it’s literally on battery manufacturing machinery.

Below is what you see when you dissect a typical cylindrical cell: wound up tightly inside the steel canister is a long, metre or more length of film. It's somewhat heavier than it looks. Unpeel it and uncoil it and you find layers of cathode and anode, and between them a polymer separator. This, ultimately, is the battery. And the key principle is: lithium ions pass from cathode to the anode as it charges. Vice versa when discharging

The cathode is where you find the lithium/cobalt/nickel/manganese etc & the vast majority of battery coverage you’ll have read focuses on it, for a understandable reasons:
- it's where the lithium is
- some of these minerals are v hard to source ethically esp cobalt
- cathodes are sexy
They're sexy because that's where a LOT of the clever battery chemistry about how much energy can be stored goes on. Cathode active materials are a really big deal but all this sexiness means we often neglect the ugly sister of the battery world: the anode.
When people in government and sometimes business ponder batteries they rarely give much thought to the anode. Why? Because unlike the cathode with its cocktail of elements, anodes are usually made almost entirely of graphite. Boring, right? Well, hold it there. Because it turns out the graphite is of more than passing importance to good battery design. The matrix of that graphite can determine how quickly lithium ions find their way across, and how long they stay there before returning.
Or, to put it another way, some graphites help a battery charge faster - much faster. Some help it hold its charge much longer. And in recent years battery makers have become v clever in the way they deploy graphite in their cells. And one key trick is to use an ever increasing amount of synthetic graphite
For it turns out there are 2 types of graphite.
- There's the kind you mine from the ground: natural or "flake" graphite.
- And the man-made kind.
And the synthetic kind is much better for fast charging. The dream of a 15 minute charging EV depends on synthetic graphite!
That takes us back to Humber. Let's go into the plant, towards the enormous drilling rigs you see here. Security is tight (you'll see why soon enough). But once in, wind your way past the spaghetti of pipes where they clean and crack the oil, towards the enormous coke drums.

Here they take the bottom of a barrel of oil - the heavy stuff which would otherwise be burnt in ship engines with v high emissions - and bake it into petroleum coke, a very pure form of carbon. They've been doing it for decades here, first with Libyan oil, then, after oil was discovered there, North Sea crude.
Up until recently this coke was mainly used in the manufacture of aluminium & in electric arc furnaces for steel. But years ago they realised they could turn this coke into an almost perfect form of graphite. This, here, is the main feedstock for synthetic graphite in your battery.

If you have an iPhone there's a significant chance the battery contains this North Sea coke in it. Similar thing if you have an electric car, depending on where the batteries are made. If you’ve got a Tesla much of the graphite is made in Japan, since that’s where Panasonic sources much of their graphite. It’s quite a revelation for those who assumed that the most controversial thing in their phone/car battery was cobalt, or lithium.
Batteries are made in part from crude oil!
In fact, there’s even more graphite in them than either of them.

That's why in charts like the second one above you'll see that our need for graphite for our future gigafactories is actually greater than our need for lithium. But cathodes are sexier than anodes so no one pays much attention. The fact that batteries are made in part from crude oil is one of the best-kept secrets of the green revolution. And while the amount of cobalt in cells is being engineered down, synthetic graphite is going the other way.

After the graphite coke is made here in Humberside it's shipped off, mostly to China, where it is turned into graphite, after which it is put into batteries. That this happens mostly in China means a lot of people think the entire graphite supply chain is already sewn up. Not so. Because it turns out it's actually quite hard to do what they do here in Humberside. There's decades of experience & IP here - so much so that a few years ago a Chinese spy tried to steal their secrets (from parent company Phillips 66's Oklahoma HQ).
Most people in the UK are completely oblivious that we have a key part of the battery supply chain on our doorstep. Downing St isn't even aware of it. Why? Maybe because when it comes to batteries anodes aren't sexy. Cathodes are sexy. Gigafactories are sexy. Lithium is sexy. But not anodes. Maybe it’s because while ministers are very happy to be pictured in pristine factories they don't like the "optics" of flame-belching oil refineries.
No matter that a molecule of oil turned into graphite is doing more to help reduce long-term emissions than most manufactured products. So the Humber Refinery carries on doing what it's doing and no one in Whitehall pays it much attention. Even though it's literally world-renowned in anode circles for the quality of the coke produced here. Or that it's the only place in Europe making this stuff.
When critical minerals committees in the US and EU (and, soon enough, the UK) make lists of key materials they tend to include natural graphite as something to keep an eye on but not synthetic, even tho it's the big growth area in batteries. Maybe because they assume it's easy to make. But, first, it’s not. Second, if one were genuinely interested in industrial strategy one would be pondering why this place ships graphite coke to China to be turned into anodes, which are then imported back as a Chinese product.
Why aren't we doing that here, in a low income corner of England? Is it because graphitisation is another high-energy high-emissions process, so doing it here would undermine our chances of getting to net zero - even though it's helping the world replace petrol cars with electric ones? Maybe. But I wouldn't discount plain ignorance either.

This goes, by the way, to a deeper point, one I've written about before. Economics is v good at aggregate statistics. It's much less good at understanding how the economy fits together. We need more maps like the one above, from the Advanced Propulsion Centre, which depicts battery supply chains.
The point isn’t necessarily about interventionism (though some would argue that given most other countries around the world explicitly or tacitly encourage certain industries, perhaps western countries should be doing so too). It’s a deeper one: the degree of understanding among policymakers about the industrial (and for that matter services sector) architecture of their economies is astoundingly primitive. Surely we should be trying harder to understand the things we are good at and bad at, and doing something about it? In a world locked in a race for batteries and battery materials it surely matters more than ever.
PS some of the above first appeared on Twitter yesterday.
The Humber Refinery in NE England.⁰This place looks and smells like a ghost of fossil fuel history. It's where they turn crude oil into petrol, jet fuel & many other petrochemical products.⁰It won't seem the obvious place to start a thread abt batteries.⁰But bear with me⁰🧵 pic.twitter.com/R9I4gR0cxD
— Ed Conway (@EdConwaySky) February 12, 2022
More to come on this in the coming weeks…




Comments ()